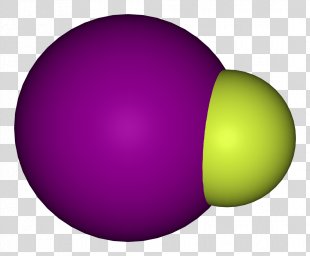
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionating to elemental iodine and iodine pentafluoride:
- 5 IF → 2 I2 IF5
However, its molecular properties can still be precisely determined by spectroscopy: the iodine-fluorine distance is 190.9 pm and the I−F bond dissociation energy is around 277 kJ mol−1. At 298 K, its standard enthalpy change of formation is ΔfH° = −95.4 kJ mol−1, and its Gibbs free energy is ΔfG° = −117.6 kJ mol−1.
It can be generated, albeit only fleetingly, by the reaction of the elements at −45 °C in CCl3F:
- I2 F2 → 2 IF
It can also be generated by the reaction of iodine with iodine trifluoride at −78 °C in CCl3F:
- I2 IF3 → 3 IF
The reaction of iodine with silver(I) fluoride at 0 °C also yields iodine monofluoride:
- I2 AgF → IF AgI
Reactions
Iodine monofluoride is used to produce pure nitrogen triiodide:
- BN 3 IF → NI3 BF3
See also
- Iodine trifluoride
- Iodine pentafluoride
- Iodine heptafluoride
References